Bristle biogenesis requires a chaetoblast-specific chitin synthase
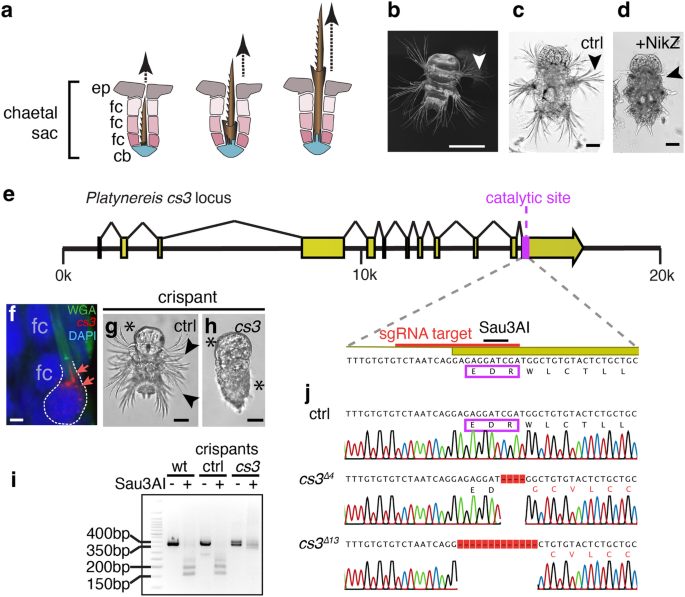
The early larvae of P. dumerilii exhibit a highly stereotypical set of compound bristles that are also referred to as spiniger-type bristles. Bristles of this type are thought to be relevant for locomotion through different substrates5,12,13. These bristles arise in specialised tissue pouches—chaetal sacs (schematised in Fig. 1a)—that line the lateral aspects of the larvae (Fig. 1b). To assess if chitin synthase activity was required for larval bristle formation, we incubated larvae prior to bristle formation in 10 µM nikkomycin Z, a competitive inhibitor of chitin synthases used across phyla14,15. In contrast to solvent-treated controls (Fig. 1c), larvae in which chitin synthase activity was blocked from 24 to 72 h post fertilisation (hpf) lacked morphologically visible bristles (Fig. 1d). These results are consistent with the notion that chitin is an essential component of bristle formation. However, they do not distinguish between the aforementioned conflicting hypotheses that chitin could either be produced by neighbouring follicle cells, or by the chaetoblasts themselves. To address this question by a genetic approach, we capitalised on prior work in our lab that had identified a specific chitin synthase gene (cs3) expressed in chaetal sacs16. Analysis of the gene and its genomic locus (Fig. 1e) confirmed that the encoded Cs3 protein contained an EDR motif (boxed in Fig. 1e) that is part of a diagnostic, evolutionarily conserved chitin synthase signature17,18. Consistently, the central aspartate of the motif is known to act as a catalytic residue in the recently characterised chitin synthase structure of a root oomycete, Phytophthora sojae19. To assess the expression of cs3 transcripts, we designed cs3-specific probes for in situ hybridisation chain reaction (in situ HCR)20. Our staining revealed specific expression of cs3 in chaetoblasts, but not in follicle cells (Fig. 1f). To test if this chaetoblast-specific chitin synthase was required for bristle formation, we turned to Cas9/CRISPR-mediated knock-out technology, which has previously been shown to work in Platynereis21. We designed a single guide RNA targeting the region encoding the EDR motif, which also contained an endogenous Sau3AI restriction site for probing genomic integrity (schematised in Fig. 1e). In contrast to a sgRNA targeting the unrelated cwo gene (ctrl in Fig. 1g), co-injection of the cs3 sgRNA with cas9 mRNA yielded embryos lacking visible bristles (Fig. 1h). Genomic amplification from wild-type specimens, cwo crispants (controls) and cs3 crispants allowed us to correlate the bristle-less phenotype with a lack of Sau3AI cleavage (Fig. 1i). Likewise, Sanger sequencing of cloned cs3 amplicons confirmed the presence of lesioned alleles—abrogating the functional EDR motif—in selected phenotypic cs3-targeted specimens, but not control crispants (Fig. 1j). These crispant analyses strongly argue that the chaetoblast-specific chitin synthase Cs3 has a key role for bristle biogenesis. This implies that the chaetoblast not only has a structural role, but itself is involved in chitin biosynthesis.
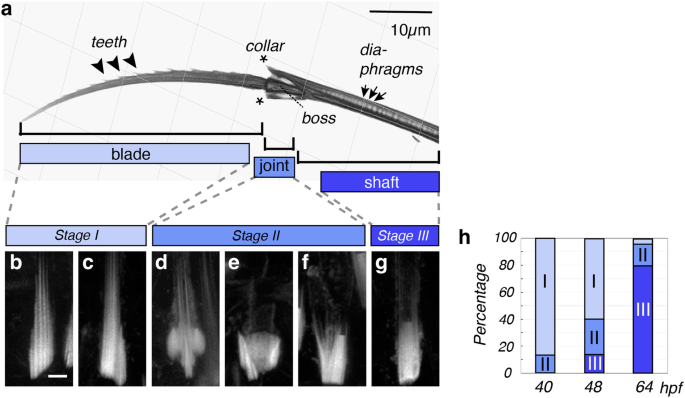
a Refractive index tomography reveals both micrometric divisions of the bristle (blade, joint, shaft) and repetitive submicrometric features (teeth, diaphragms). b–g Correlated geometries of chaetoblast surfaces, as analysed by Phalloidin488 labelling of F-actin in the microvilli of chaetoblasts at synthesis stages I, II and III. Images are Huygen-deconvoluted Z-stack maximal projections of apical chaetoblast surfaces, with distal sides pointing to the top. Scale bar: 2 µm. h Distribution of stages represented in (b–g) during the development of bristles at 40, 48 and 72 h post fertilisation (hpf). Source data are provided as a Source Data file. Scale bars a 10 µm; b 2 µm.
Biogenesis of stereotypical bristle structures is matched by distinct microvillar geometries
To characterise the spatial properties of larval bristles at higher resolution, and deduce the temporal time scales required for their production, we next established a biochemical isolation protocol separating bristles from the surrounding tissue, and employed refractive index tomography on isolated bristles. Tomograms allowed us to visualise diagnostic features of larval bristles on different size scales: they show the tripartite subdivision of the bristle into a micrometre-scale blade, joint and shaft (Fig. 2a and Supplementary Movie 1), and reveal submicrometric features, such as the teeth of the blade, the collar and boss of the joint, and the diaphragms inside the shaft (Fig. 2a). The teeth were consistently aligned on the exterior curvature of the blade. In proximity of the joint, the distance between adjacent teeth measured ~2.5 µm. Towards the tip of the blade, this distance gradually declined, reaching 0.4–1.3 µm at the distal tip. As the formation of a blade requires around 12 h and during that time produces a total of 21 teeth (s.b.), a new tooth is, on average, initiated every ~35 min. In the shaft, diaphragms were regularly spaced at ~0.7 µm distance, indicating that a new diaphragm was deposited every ~12 min. Taken together, our quantification not only supports the idea that the deposition of blades, joints, and shafts is driven by changes in the biogenesis programme on the scale of hours/days, but that additional dynamics on the scale of minutes/hours are responsible for depositing repetitive, submicrometric features of the blade and shaft.
The stereotypicity of bristle structures in Platynereis, as well as the documented species-specific variations of bristle patterns in other polychaetes4,5 indicate that bristle biogenesis is driven by a genetically programmed cellular mechanism. In other contexts, such as the vertebrate hair cells or lateral line neuromasts, elaborate cellular geometries rely on the occurrence of specialised apical microvilli22. Consistent with the role of microvilli in polychaete bristle biogenesis, classical ultrastructural work has found cellular protrusions to be associated with bristles in the annelid Nereis vexillosa7, and recent analyses have confirmed largely similar geometries in chaetoblasts of Platynereis individuals fixed at different stages of development9. To systematically probe for a correlation of microvillar geometry at the cell cortex of Platynereis chaetoblasts and the biogenesis of diagnostic bristle features, we performed a systematic analysis of chaetoblast microvilli at distinct times of development associated with the production of characteristic bristle features. As microvilli are usually characterised by filamentous actin (F-actin) fibres, we used the F-actin-binding molecule phalloidin to assess microvillar geometries.
For our analyses, we chose larvae fixed at 40, 48 or 64 hpf, which are developmental stages at which blade/teeth, joint, and shaft are formed, respectively. Phalloidin-based visualisation of actin in ~2400 chaetoblasts by confocal microscopy revealed distinct geometries of microvillar patterns in chaetoblasts that we could assign to the timeline of bristle biogenesis (Fig. 2b, c): At stage I, when the blade with the teeth is forming, we consistently observe a set of ~10 microvilli of different length, collectively forming a cone-shaped assembly (Fig. 2b). This assembly is eventually accompanied by a slim side group of microvilli that are also aligned parallel to each other (Fig. 2c). A diagnostic feature of stage II is the occurrence of a crescent-shaped microvillar arrangement that surrounds a cone formed by parallel microvilli (Fig. d). Progressively, the crescent shape forms a cup-shaped object (Fig. 2e). In a subset of stage II patterns, the cup-shaped object includes a larger, flat-tipped microvillus on one side (Fig. 2f). Stage III is characterised by the presence of a central, flat-tipped axial microvillus of 0.5−0.8 µm diameter (Fig. 2g), flanked by a group of paraxial annular microvilli. The frequency of the different pattern categories by developmental stage (Fig. 2h) not only supports the aforementioned timeline, but also attests to a high degree of synchronicity in the development of individual compound bristles within the developing larva. To test if the formation of microvilli depended on chitin synthesis, we assessed actin geometries after NikZ-mediated inhibition of chitin synthesis and in bristle-less cs3 crispants. In both cases, microvilli were consistently present, even though the fine morphology of the microvillar assembly exhibited abnormalities (Supplementary Figs. 1, 2). Taken together, our analyses support the notion that stereotypic arrangements of a dynamic microvillar programme are correlated with individual steps of Platynereis bristle formation.
A mechanistic model for microvillar-based printing of submicrometric bristle features
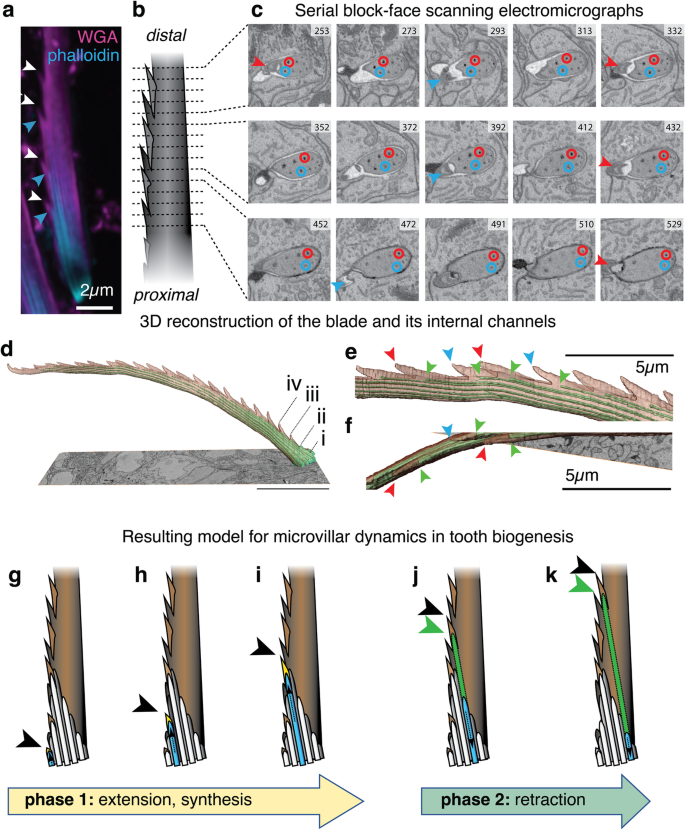
We next assessed to which degree the presence of individual microvilli could be traced to fine details of the produced bristle. Light-microscopic analyses suggested that individual microvilli in stage I arrangements could be associated with the formation of individual teeth (Fig. 3a). To examine the details of this potential correlation in more detail, and gain insight into the question how a rather constant set of ~10 microvilli produces twice as many teeth, we turned to serial block-face scanning electron microscopy (SBF-SEM), a technique able to reconstruct micrometric objects from serial electron micrographs sampled at nanometric distance23. For analysing the relationship between teeth and microvillar arrangements, we investigated 32 chaetoblast samples at 48 hpf, when blade production should be mostly completed. Machine-assisted modelling of more than 1000–40 nm slices of a representative chaetoblast (Fig. 3b, c and Supplementary Movie 2), followed by manual polishing, allowed us to reconstruct a 46.4 µm blade with 21 teeth (Fig. 3d–f and Supplementary Movie 3). The inside of the SBF-SEM-based blade model is characterised by longitudinal channels. According to early work in nereidid polychaetes6,7, only channels close to the chaetoblast contain the microvilli observed in the aforementioned analysis, whereas the distal channels result from the disassembly of microvilli after the solidification of the chitin matrix (Fig. 3c and Supplementary Movie 2). Tracking these channels across the distal-proximal axis revealed that they were neither continuous nor exactly aligned with the main axis of the blade. Rather, individual channels had lengths of around 10–20 µm. Their distal-most tips were positioned close to the serrated edge, while they ended close to the opposite edge of the blade (see circles in Fig. 3c). Spacing and size of teeth in the SBF-SEM-based reconstruction matched our light-microscopic observations. Each tooth was accompanied by a channel that started at a mean distance of ~1.6 µm from the tooth tip (Fig. 3e). The orientation of teeth at the outer edge of the blade alternated in a regular fashion, pointing either to the left or the right face of the blade (Fig. 3c, f and Supplementary Movie 3). Consistent with the notion that each tooth was produced by a single microvillus, the channel tips on the outer edge of the blade exhibited the same left/right alternation that we observed for the teeth (Fig. 3c, f and Supplementary Movie 3). Moreover, the channels tips at the base of the bristle (Fig. 3d i–iv) exhibited a gradual increase in the length of the respective tooth that is also detectable in light-microscopic analyses (Fig. 3a).
a Co-visualisation of chitin (WGA) and F-actin (phalloidin) suggests a correlation of individual microvilli (blue arrowheads) with individual teeth (red arrowheads); b–f SBF-SEM analysis; b Overview scheme and c selected (numbered) ~40 nm cross sections obtained by SBF-SEM on a synthesised blade (see Supplementary Movie 2). Arrowheads demarcate the chitin tip of left (red) or right (blue) teeth appearing prior to the occurrence of the corresponding channel. Red and blue circles trace two of the channels across the represented stack, revealing a systematic displacement towards the non-serrated edge, where older channels are discontinued. d–f Overview and details of the resulting 3D reconstruction (cf. Supplementary Movie 3) of the reconstructed blade; red and blue arrowheads demarcate tooth tips, green arrowheads demarcate tips of corresponding channels; in (d), (i–iv) demarcate the first four teeth that exhibit an increase in length. g–k Resulting model for the synthesis of a given tooth, fitting the triangle-shaped arrangement of microvilli (dark/light grey) observed in Fig. 2b, c into the base of the bristle. The model predicts that extension (phase 1) is associated with active chitin biosynthesis/deposition (yellow); subsequent disassembly of actin (phase 2) would leave the characteristic channels (schematised in green). Arrowheads demarcate tooth tips (black) and tips of channels (green). The direction of bristle growth is indicated by brown, dashed arrows.
As the channels in the bristle matrix must reflect the respective orientation and dynamics of the microvillar assembly during blade synthesis, these data allow us to propose a first mechanistic model of how individual teeth are engineered during bristle biogenesis (Fig. 3g–k). This model predicts that each microvillus undergoes an extension phase 1 (Fig. 3g–i), during which chitin biosynthesis close to its tip leads to the growth of a new tooth. After chitin synthesis ceases, a disassembly phase 2 (Fig. 3j, k) would leave the characteristic channels in the chitin matrix. Systematic displacement of old microvilli by newly added microvilli at the serrated edge would push the disassembling microvilli to the opposite end of the triangular microvillar assembly over time.
Using the same SBF-SEM strategy, we also investigated and reconstructed the internal structure and channel orientation of larvae sampled during joint and shaft formation (Supplementary Figs. 3, 4 and Supplementary Movies 4–7, respectively). These analyses revealed additional details about changes in microvillar geometries that inform the conformations observed in our light-microscopic analyses (Fig. 2). For instance, our analyses uncovered that the axial microvillus of the shaft originates from a central microvillus in the boss of the joint that grows dramatically in diameter (ac/amv in Supplementary Fig. 3) to finally occupy the majority of the shaft (Supplementary Fig. 4). By contrast, some of the microvilli surrounding the nascent axial microvillus in the boss gradually repositioned to form slim, tapered annular microvilli around the circumference of the axial microvillus at the shaft base (Supplementary Fig. 4).
Taken together, both our light- and electron-microscopic analyses support a tight connection between the different microvillar geometries of the chaetoblast at distinct stages, and the production of micrometric and submicrometric features of the respective bristle. For the production of teeth, the triangular geometry of microvilli, as well as the details of the SBF-SEM-based model, are consistent with a printing mechanism in which new microvilli spawn at one edge of the blade, and extend for four or more “cycles” while depositing chitin close to their apex, before starting to disassemble again, leaving a channel in the solidified chitin matrix.
Changes in F-actin polymerisation rate impact on feature deposition
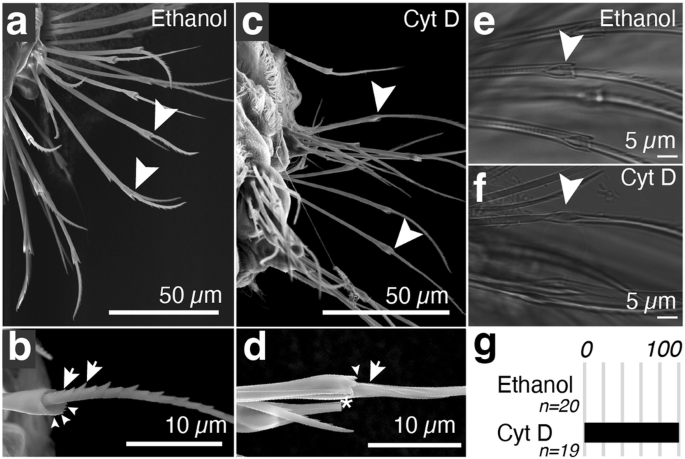
Such a printing mechanism would predict that microvillar extension is required for depositing teeth. To test this notion, we decided to use a small molecule approach. Cytochalasins are fungal metabolites that bind to barbed or fast-growing ends of microfilaments and reduce the addition and dissociation of monomeric actin at the barbed end24. They have been shown to interfere with microvillar actin polymerisation in intestinal epithelial brush border preparations25, and were already employed to probe actin-dependent processes in Platynereis development26. We treated larvae at 40 hpf with 10 µM cytochalasin D, then washed off the drug at 48 hpf and let larvae develop until fixation at 72 hpf. Specimens were imaged using a field emission gun—SEM to assess bristle morphology. Control specimens (treated with 0.1% ethanol) exhibited normal bristle morphology (Fig. 4a), with teeth positioned along the blade (arrow Fig. 4b). By contrast, in cytochalasin D-treated specimens, we observed that the proximal half of the blade (synthesised during treatment) lacked teeth and appeared smooth (Fig. 4c arrow, d). We noted that the chosen time window also had effects on joint morphology, leading to a smaller opening of the joint, and a partial reduction of dentate substructures in the crown (arrowheads Fig. 4b, d). DIC microscopy (Fig. 4e, f and Supplementary Fig. 5) allowed us to quantify these effects: All of the control animals (n = 20) showed teeth and a properly formed boss (Fig. 4e, g). Cytochalasin D-treated larvae (n = 19) exhibited a lack of teeth, and a smooth and oval shaft (Fig. 4f, g). Cytochalasin D treatment thus impairs the formation of the teeth and joint features, at a time window (40–48 hpf) when these structures are typically produced. Taken together, our results support that the dynamicity of F-actin is required for the production of micro- and submicrometric bristle features, revealing a critical component of morphogenesis at this largely unexplored size scale.
a, b SEM Images of larvae that were treated with vehicle (ethanol 0.1%); c, d corresponding larvae treated with 10 µM cytochalasin D from 40 hpf to 48 hpf. Images were taken at 72 hpf. Arrowheads point to the joint region of the bristle; arrows point to teeth (control) or the corresponding, smooth section on blades synthesised during cytochalasin treatment. e, f DIC images of bristles present on larvae that were treated with vehicle (e) or cytochalasin D (f). g Percentage of larvae with defects in bristle morphology after treatment with vehicle or cytochalasin D. Source data are provided as a Source Data file.